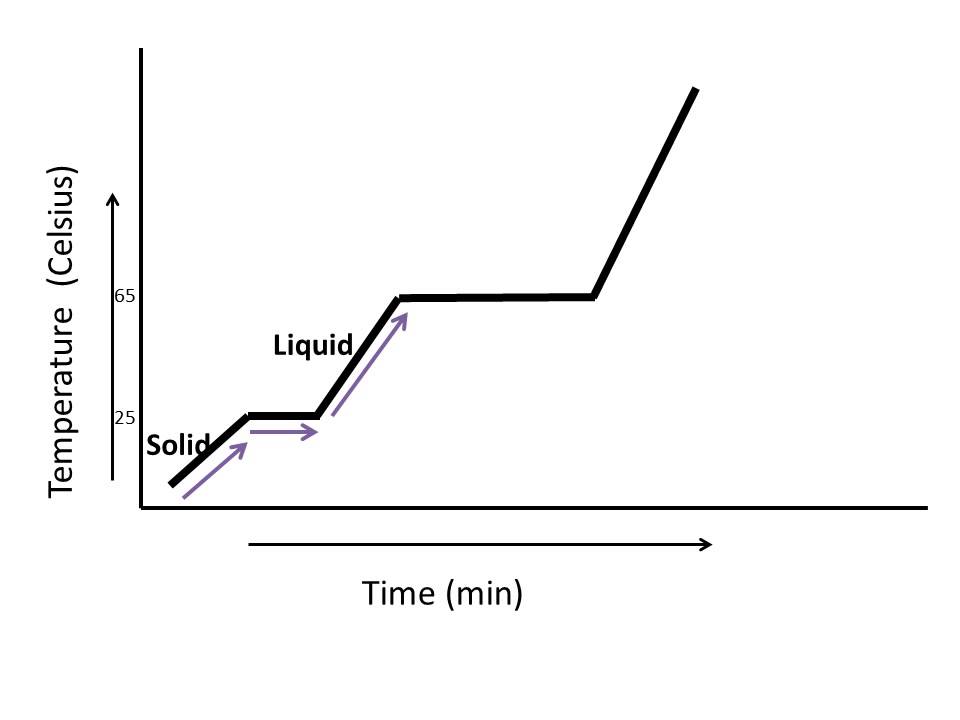
Heating Curve Of Water Examples . For heating curves, we start with a solid and add heat energy. Heating and cooling curves are graphs. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: The heating curve for water illustrates the relationship between temperature and heat energy as water undergoes phase changes. The diagram below shows the heating curve for water. Use the heat of vaporization (. The heating curve for water shows how the temperature of a given quantity of water changes. Δ h v a p \delta h_ {vap} δhvap. Liquid water becomes water vapor or steam when it enters the gaseous phase. The temperature of the system is plotted as function of time. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Heat is flowing at a constant rate, thus time can be interpreted as.
from www.youtube.com
This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Δ h v a p \delta h_ {vap} δhvap. The diagram below shows the heating curve for water. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Heat is flowing at a constant rate, thus time can be interpreted as. The temperature of the system is plotted as function of time. For heating curves, we start with a solid and add heat energy. The heating curve for water shows how the temperature of a given quantity of water changes. Liquid water becomes water vapor or steam when it enters the gaseous phase. Use the heat of vaporization (.
How to Read a Heating Curve YouTube
Heating Curve Of Water Examples Liquid water becomes water vapor or steam when it enters the gaseous phase. Heating and cooling curves are graphs. Heat is flowing at a constant rate, thus time can be interpreted as. Δ h v a p \delta h_ {vap} δhvap. For heating curves, we start with a solid and add heat energy. The diagram below shows the heating curve for water. Use the heat of vaporization (. Liquid water becomes water vapor or steam when it enters the gaseous phase. The temperature of the system is plotted as function of time. The heating curve for water shows how the temperature of a given quantity of water changes. The heating curve for water illustrates the relationship between temperature and heat energy as water undergoes phase changes. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water.
From lessonzoneyvonne.z13.web.core.windows.net
Heating Curve Of Water Worksheet Heating Curve Of Water Examples Liquid water becomes water vapor or steam when it enters the gaseous phase. Heat is flowing at a constant rate, thus time can be interpreted as. The heating curve for water shows how the temperature of a given quantity of water changes. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm. Heating Curve Of Water Examples.
From studylib.net
Heating Curve of Water Heating Curve Of Water Examples For heating curves, we start with a solid and add heat energy. Use the heat of vaporization (. Heating and cooling curves are graphs. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: The heating curve for water illustrates the relationship. Heating Curve Of Water Examples.
From www.youtube.com
Heating Curve of Water YouTube Heating Curve Of Water Examples Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. For heating curves, we start with a solid and add heat energy. Liquid water becomes water vapor or steam when it enters the gaseous phase. Use the heat of vaporization (. The diagram below shows the heating curve for water.. Heating Curve Of Water Examples.
From ar.inspiredpencil.com
Specific Heat Of Water Chart Heating Curve Of Water Examples For heating curves, we start with a solid and add heat energy. Heating and cooling curves are graphs. Use the heat of vaporization (. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: The diagram below shows the heating curve for. Heating Curve Of Water Examples.
From lessonfullantje.z19.web.core.windows.net
Draw And Label The Heating Curve For Water Heating Curve Of Water Examples Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Liquid water becomes water vapor or steam when it enters the gaseous phase. The heating curve for water illustrates the relationship between temperature and heat energy as water undergoes phase changes. The temperature of the system is plotted as function. Heating Curve Of Water Examples.
From ar.inspiredpencil.com
Graph Heating Curve For Water Heating Curve Of Water Examples Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The temperature of the system is plotted as function of time. Liquid water becomes water vapor or steam when it enters the gaseous phase. Use the heat of vaporization (. The heating curve for water illustrates the relationship between temperature. Heating Curve Of Water Examples.
From scienceisntscary.wordpress.com
Boiling point Ease Into Science Heating Curve Of Water Examples The heating curve for water illustrates the relationship between temperature and heat energy as water undergoes phase changes. Heating and cooling curves are graphs. Heat is flowing at a constant rate, thus time can be interpreted as. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. For heating curves,. Heating Curve Of Water Examples.
From www.researchgate.net
Heating curve for water. Download Scientific Diagram Heating Curve Of Water Examples Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. For heating curves, we start with a solid and add heat energy. Heat is flowing at a constant rate, thus time can be interpreted as. The heating curve for water illustrates the relationship between temperature and heat energy as water. Heating Curve Of Water Examples.
From kimyachi.blogspot.com
Kimyaçı Phase Changes Energy Changes Heating Curves Heating Curve Of Water Examples The heating curve for water shows how the temperature of a given quantity of water changes. Liquid water becomes water vapor or steam when it enters the gaseous phase. Heat is flowing at a constant rate, thus time can be interpreted as. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm. Heating Curve Of Water Examples.
From www.docsity.com
THE HEATING CURVE OF WATER Slides Chemistry Docsity Heating Curve Of Water Examples This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: For heating curves, we start with a solid and add heat energy. Heating and cooling curves are graphs. Use the heat of vaporization (. Heat is flowing at a constant rate, thus. Heating Curve Of Water Examples.
From learningfullmaurer.z1.web.core.windows.net
Heating Curve Of Water Diagram Heating Curve Of Water Examples Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The diagram below shows the heating curve for water. For heating curves, we start with a solid and add heat energy. The temperature of the system is plotted as function of time. Use the heat of vaporization (. The heating. Heating Curve Of Water Examples.
From chem.libretexts.org
11.7 Heating Curve for Water Chemistry LibreTexts Heating Curve Of Water Examples Use the heat of vaporization (. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: For heating curves, we start with a solid and add heat energy. Δ h v a p \delta h_ {vap} δhvap. The heating curve for water. Heating Curve Of Water Examples.
From www.researchgate.net
Heating curve of water at fixed atmospheric pressure. It corresponds to Heating Curve Of Water Examples Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. The heating curve for water shows how the temperature of a given quantity of water changes. Use the heat of vaporization (. Δ h v a p \delta h_ {vap} δhvap. Heat is flowing at a constant rate, thus time. Heating Curve Of Water Examples.
From www.slideserve.com
PPT Heating Curve for Water PowerPoint Presentation, free download Heating Curve Of Water Examples This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: The diagram below shows the heating curve for water. The temperature of the system is plotted as function of time. The heating curve for water shows how the temperature of a given. Heating Curve Of Water Examples.
From www.gauthmath.com
The figure below shows the heating curve of water with four labeled Heating Curve Of Water Examples The diagram below shows the heating curve for water. Liquid water becomes water vapor or steam when it enters the gaseous phase. The heating curve for water shows how the temperature of a given quantity of water changes. Heating and cooling curves are graphs. Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75. Heating Curve Of Water Examples.
From www.chegg.com
Solved The Figure shown below is the heating curve obtained Heating Curve Of Water Examples Liquid water becomes water vapor or steam when it enters the gaseous phase. The heating curve for water shows how the temperature of a given quantity of water changes. The temperature of the system is plotted as function of time. The heating curve for water illustrates the relationship between temperature and heat energy as water undergoes phase changes. Heating and. Heating Curve Of Water Examples.
From mavink.com
What Is A Heating Curve Heating Curve Of Water Examples Use the heat of vaporization (. For heating curves, we start with a solid and add heat energy. Heating and cooling curves are graphs. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: The diagram below shows the heating curve for. Heating Curve Of Water Examples.
From www.youtube.com
Heating Curve for Water YouTube Heating Curve Of Water Examples This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Figure 11.4.1 shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Δ h v a p \delta h_ {vap} δhvap. The temperature of. Heating Curve Of Water Examples.